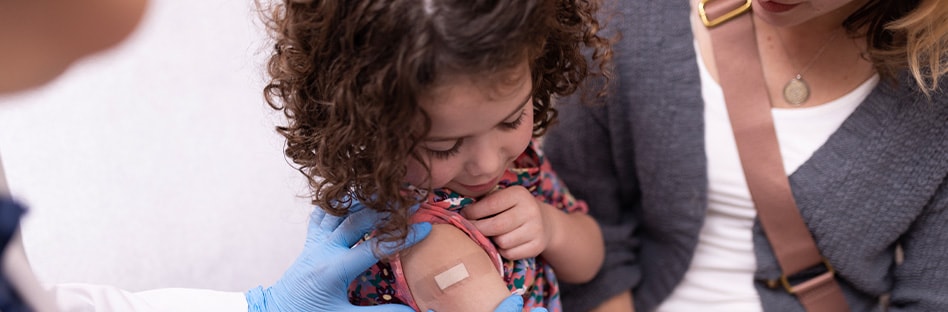
The COVID-19 Vaccine†
Vaccination is an effective strategy to lessen the incidence of COVID-19 infections, especially those that cause severe illness, hospitalization and death. The COVID-19 vaccine is currently available, depending on the manufacturer, for individuals 6 months and older. The CDC recommends the updated COVID-19 vaccine to provide broader immune protection against the current variant.
At-Home COVID-19 Testing Solutions
Essential Supplies
Loading Content
Tools & Resources
†The Pfizer vaccine has been approved by FDA for use in individuals 16 years of age and older. For individuals age 6 months – 15 years old, this product has not been approved or licensed by FDA, but has been authorized for emergency use by FDA, under an EUA to prevent Coronavirus Disease 2019 (COVID-19). The emergency use of this product is only authorized for the duration of the declaration that circumstances exist justifying the authorization of emergency use of the medical product under Section 564(b)(1) of the FD&C Act unless the declaration is terminated or authorization revoked sooner.
The Moderna vaccines have not been approved or licensed by FDA, but have been authorized for emergency use by FDA, under an EUA to prevent Coronavirus Disease 2019 (COVID-19) for use in individuals 6 months of age and older; and the emergency use of these products is only authorized for the duration of the declaration that circumstances exist justifying the authorization of emergency use of the medical products under Section 564(b)(1) of the FD&C Act unless the declaration is terminated or authorization revoked sooner.
The molecular, antigen, and antibody (collectively, “Tests”) have not been FDA cleared or approved
All Tests have been authorized by FDA under EUAs for use by authorized laboratories or are CLIA-waived tests;
The molecular tests have been authorized only for the detection of nucleic acid from SARS-CoV-2, not for any other viruses or pathogens;
The antigen tests have been authorized only for the detection of proteins from SARS-CoV-2, not for any other viruses or pathogens;
The antibody tests have been authorized only for the presence of IgM and IgG antibodies against SARS-CoV-2, not for any other viruses or pathogens; and,
Tests are only authorized for the duration of the declaration that circumstances exist justifying the authorization of emergency use of in vitro diagnostics for detection and/or diagnosis of COVID-19 under Section 564(b)(1) of the Act, 21 U.S.C. § 360bbb-3(b)(1), unless the authorization is terminated or revoked sooner.
Review our Pharmacy Notice of Nondiscrimination to learn more about how The Kroger Co.’s and its subsidiaries’ pharmacies and retail health clinics (“Kroger”) complies with applicable Federal civil rights laws.
Pharmacy, Clinic, and Nutrition services are available in select areas. Access our pharmacy locator to find a pharmacy near you. The Little Clinic practices in the following states only: AZ, KY, OH, TN, CO, IN, GA, KS, VA. Access our clinic locator to find a clinic near you. Nutrition services are not available in AK, MT, NJ, NY, SC, WY, or where otherwise prohibited by applicable law. Walk-ins welcome as time allows.